Draw the electron configuration for a neutral atom of titanium – Delving into the electron configuration of a neutral titanium atom, we embark on a journey to unravel the fundamental building blocks of matter. This exploration will provide a deeper understanding of titanium’s atomic properties and its role in shaping its chemical behavior.
As we delve into the intricacies of titanium’s electron configuration, we will uncover the significance of atomic number, the Aufbau principle, and Hund’s rule. These concepts will guide us in constructing an orbital diagram that visually represents the arrangement of electrons within titanium’s atomic structure.
Electron Configuration Overview
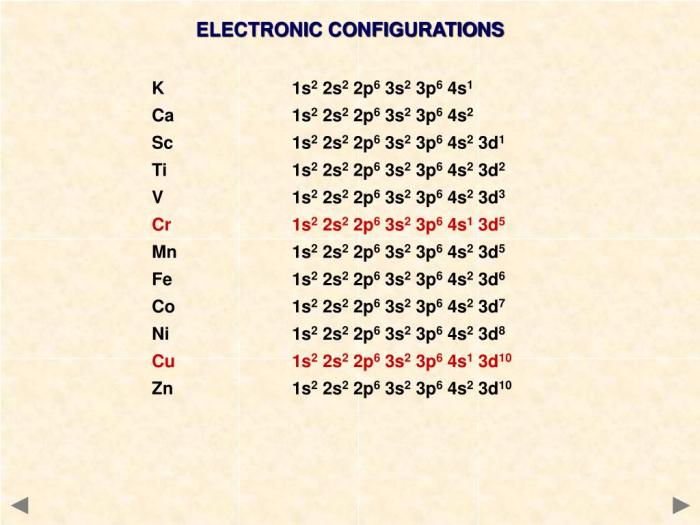
Electron configuration refers to the distribution of electrons in the atomic orbitals of an atom. It provides crucial insights into the chemical properties and behavior of elements. Electron configuration is determined by the atomic number, which represents the number of protons in the nucleus.
Titanium’s Electron Configuration
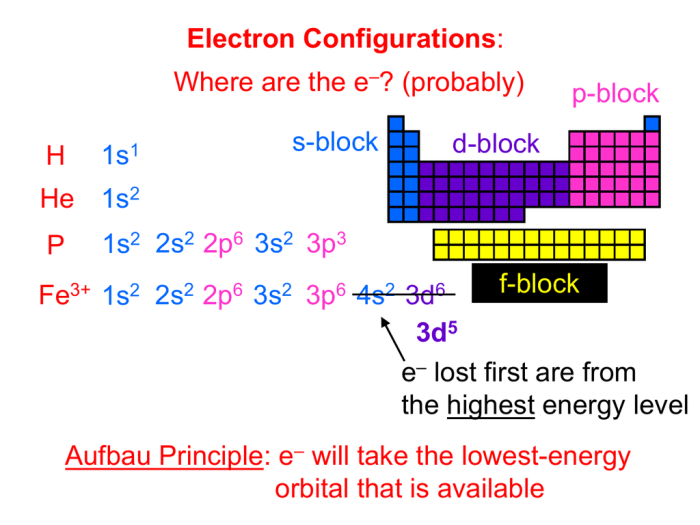
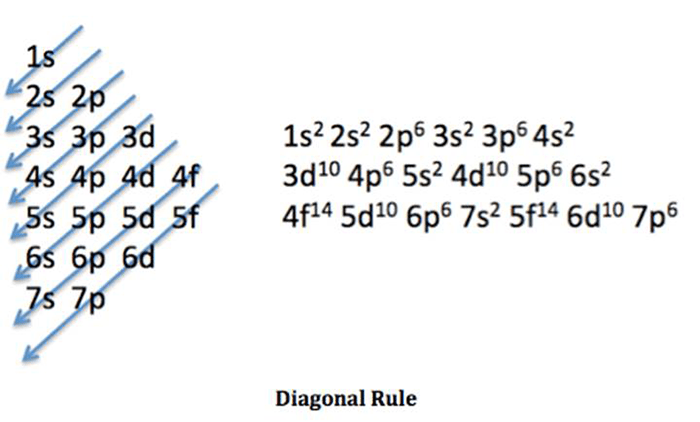
Titanium (Ti) has an atomic number of 22, indicating the presence of 22 protons in its nucleus. According to the Aufbau principle, electrons fill atomic orbitals in the order of increasing energy levels and sublevels. The electron configuration of a neutral titanium atom is:
s22s 22p 63s 23p 63d 24s 2
Orbital Diagram Representation
An orbital diagram provides a visual representation of the electron configuration. Each orbital is depicted as a box, with arrows representing the electrons. The orbital diagram for titanium is:

The Aufbau principle dictates the filling order, while Hund’s rule governs the arrangement of electrons within orbitals. Hund’s rule states that orbitals of equal energy are filled with unpaired electrons before pairing occurs.
Valence Electrons and Chemical Properties: Draw The Electron Configuration For A Neutral Atom Of Titanium
Valence electrons are the electrons in the outermost energy level. Titanium has two valence electrons, located in the 4s orbital. Valence electrons play a significant role in determining the chemical properties of an element. Titanium’s two valence electrons allow it to form stable bonds with other elements, making it a relatively reactive metal.
FAQ Corner
What is the electron configuration of a neutral titanium atom?
The electron configuration of a neutral titanium atom is 1s 22s 22p 63s 23p 63d 24s 2.
How many valence electrons does titanium have?
Titanium has two valence electrons.
What is the significance of titanium’s electron configuration?
Titanium’s electron configuration helps explain its chemical properties, such as its high strength, corrosion resistance, and ability to form various alloys.

