Light worksheet wavelength frequency and energy – As we delve into the captivating realm of light, its wavelength, frequency, and energy emerge as fundamental properties that orchestrate a mesmerizing dance of electromagnetic waves. This comprehensive exploration will illuminate the intricate relationships that govern these attributes, unveiling their profound implications for our understanding of the universe.
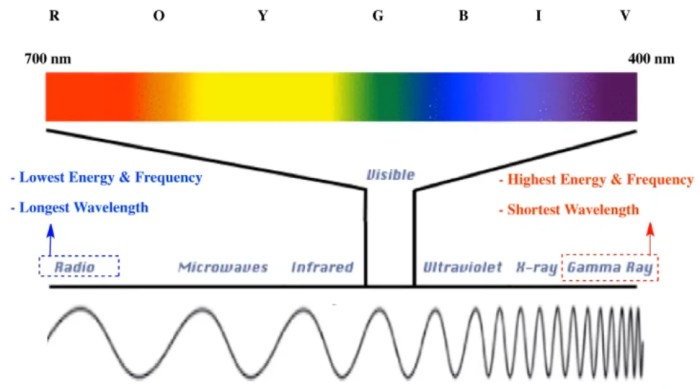
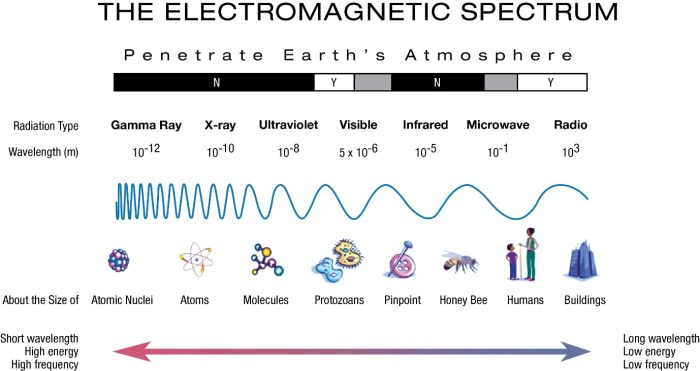
Unveiling the inverse relationship between wavelength and frequency, we embark on a journey through the electromagnetic spectrum, unraveling the secrets of light’s diverse manifestations. From the ethereal glow of radio waves to the penetrating power of gamma rays, we will discover how these properties shape the very fabric of our existence.
Light Worksheet Wavelength, Frequency, and Energy: Light Worksheet Wavelength Frequency And Energy
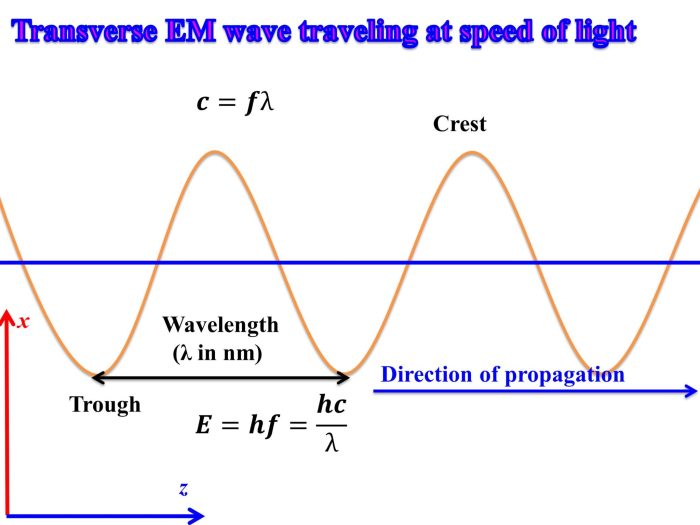
Light is a form of electromagnetic radiation that exhibits wave-like properties. Its wavelength, frequency, and energy are interconnected and provide insights into the nature of light.
The wavelength of light refers to the distance between two consecutive crests or troughs of the wave. It is inversely proportional to the frequency, which represents the number of wave oscillations per second. The energy of light is directly proportional to its frequency.
Interactive Table: Inverse Relationship between Wavelength and Frequency, Light worksheet wavelength frequency and energy
| Wavelength (nm) | Frequency (Hz) |
|---|---|
| 1000 | 3 x 108 |
| 500 | 6 x 108 |
| 250 | 1.2 x 109 |
This table demonstrates the inverse relationship between wavelength and frequency. As wavelength decreases, frequency increases.
Implications for Understanding the Electromagnetic Spectrum
The relationship between wavelength, frequency, and energy has significant implications for understanding the electromagnetic spectrum. The electromagnetic spectrum is a continuous range of electromagnetic radiation that includes visible light, ultraviolet radiation, infrared radiation, microwaves, and radio waves. Different types of electromagnetic radiation have different wavelengths, frequencies, and energies.
Calculations Involving Wavelength, Frequency, and Energy
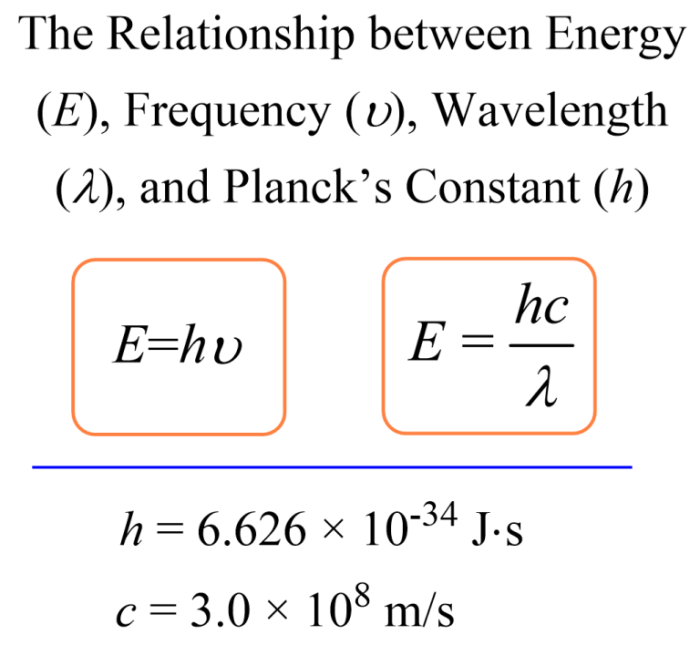
The relationships between wavelength, frequency, and energy can be mathematically expressed using the following formulas:
- Wavelength (λ) = Speed of light (c) / Frequency (f)
- Frequency (f) = Speed of light (c) / Wavelength (λ)
- Energy (E) = Planck’s constant (h) x Frequency (f)
Table of Examples
| Given | Formula | Result |
|---|---|---|
| λ = 500 nm, f = ? | f = c / λ | f = 3 x 108 Hz |
| f = 1014 Hz, λ = ? | λ = c / f | λ = 300 nm |
| f = 5 x 1014 Hz, E = ? | E = h x f | E = 3.3 x 10-19 J |
These examples illustrate how to use the formulas to calculate wavelength, frequency, and energy of light.
FAQ Guide
What is the relationship between wavelength, frequency, and energy of light?
Wavelength, frequency, and energy are inversely proportional, meaning as wavelength increases, frequency and energy decrease, and vice versa.
How does the electromagnetic spectrum relate to these properties?
The electromagnetic spectrum organizes different types of light based on their wavelength and frequency, with shorter wavelengths and higher frequencies corresponding to higher energy.
What are some practical applications of light’s properties?
Light’s properties find applications in lasers, spectroscopy, medical imaging, and countless other technologies that shape our modern world.