Identify the approximate bond angles in each molecule. This guide delves into the fascinating realm of molecular geometry, where we uncover the secrets of bond angles using the powerful tool of VSEPR theory. Prepare to embark on an enlightening journey as we explore the intricate dance of atoms and unravel the mysteries of their spatial arrangements.
VSEPR theory, an acronym for Valence Shell Electron Pair Repulsion theory, provides a conceptual framework for understanding the three-dimensional structures of molecules. By considering the repulsive forces between electron pairs, we can predict the molecular geometry and, consequently, the bond angles between atoms.
This understanding forms the cornerstone of our exploration into the molecular world.
VSEPR Theory and Molecular Geometry: Identify The Approximate Bond Angles In Each Molecule.
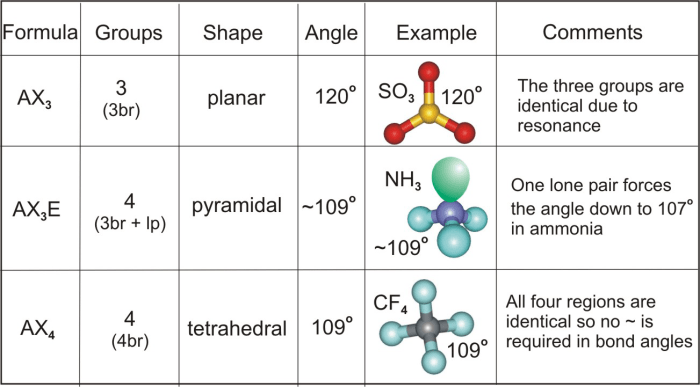
VSEPR (Valence Shell Electron Pair Repulsion) theory is a model used to predict the geometry of molecules based on the repulsion between electron pairs in the valence shell of the central atom.
VSEPR theory states that electron pairs in the valence shell of an atom will arrange themselves in a way that minimizes the repulsion between them. This arrangement determines the molecular geometry of the molecule.
Examples of Molecular Geometries, Identify the approximate bond angles in each molecule.
- Linear: 2 electron pairs, 180° bond angle (e.g., CO 2)
- Trigonal planar: 3 electron pairs, 120° bond angle (e.g., BF 3)
- Tetrahedral: 4 electron pairs, 109.5° bond angle (e.g., CH 4)
- Octahedral: 6 electron pairs, 90° bond angle (e.g., SF 6)
Bond Angles in Different Molecular Geometries
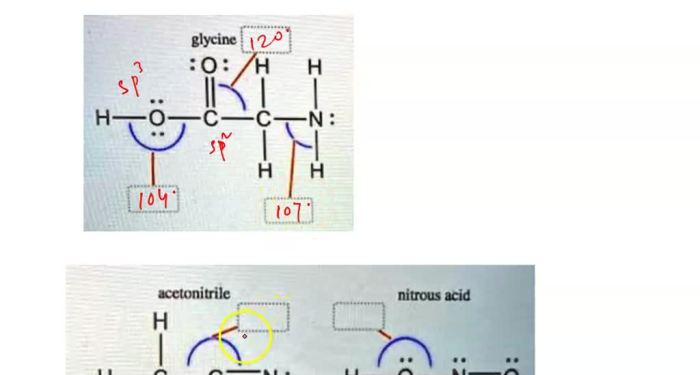
The bond angles in different molecular geometries are determined by the number of electron pairs in the valence shell of the central atom.
In linear molecules, the two electron pairs are arranged on opposite sides of the central atom, resulting in a bond angle of 180°. In trigonal planar molecules, the three electron pairs are arranged in a plane around the central atom, resulting in bond angles of 120°. In tetrahedral molecules, the four electron pairs are arranged in a tetrahedron around the central atom, resulting in bond angles of 109.5°. In octahedral molecules, the six electron pairs are arranged in an octahedron around the central atom, resulting in bond angles of 90°.
| Molecular Geometry | Bond Angle |
|---|---|
| Linear | 180° |
| Trigonal planar | 120° |
| Tetrahedral | 109.5° |
| Octahedral | 90° |
Exceptions to VSEPR Theory
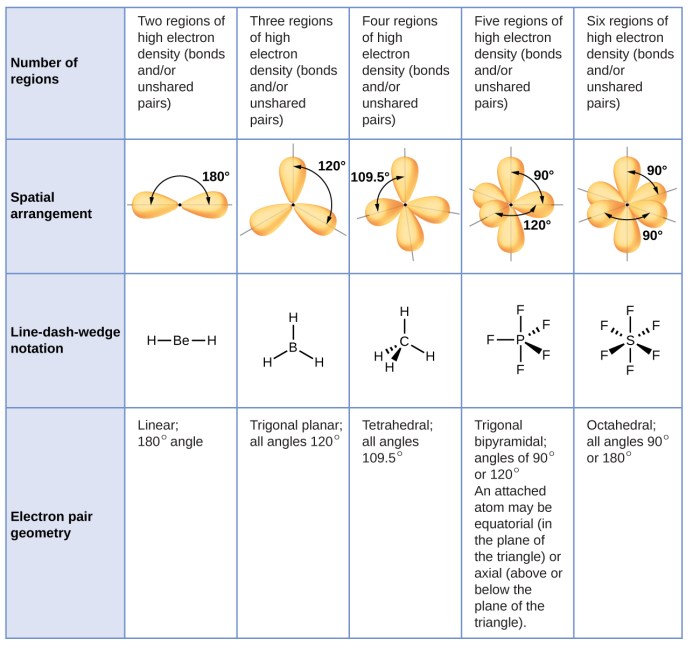
VSEPR theory is a useful tool for predicting the molecular geometry of molecules, but there are some exceptions to the theory.
One exception is steric hindrance. Steric hindrance occurs when the atoms in a molecule are too close together, which can cause the molecule to adopt a different geometry than predicted by VSEPR theory. For example, the molecule PCl 5has a trigonal bipyramidal geometry instead of the octahedral geometry predicted by VSEPR theory because the chlorine atoms are too close together to fit in an octahedron.
Another exception to VSEPR theory is resonance. Resonance occurs when a molecule has multiple Lewis structures, which means that the electrons in the molecule are delocalized. Delocalized electrons do not experience the same repulsion as localized electrons, which can lead to a different molecular geometry than predicted by VSEPR theory.
For example, the molecule benzene has a hexagonal geometry instead of the tetrahedral geometry predicted by VSEPR theory because the electrons in the molecule are delocalized.
Applications of Bond Angle Analysis
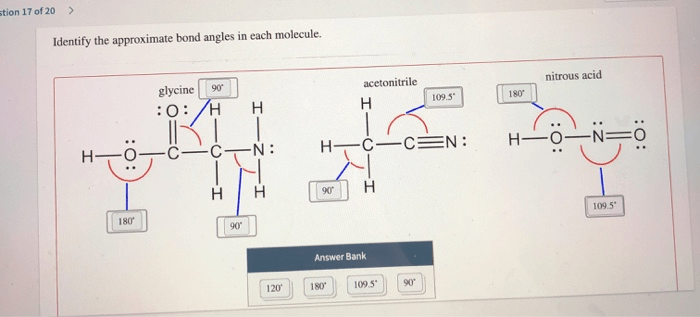
Bond angle analysis can be used to determine the molecular structure of a molecule. By measuring the bond angles in a molecule, it is possible to determine the molecular geometry of the molecule. This information can then be used to predict the properties of the molecule.
Bond angle analysis has also been used to study the interactions between molecules. By measuring the bond angles in a molecule, it is possible to determine the strength of the interactions between the molecules. This information can be used to design new materials and to understand the behavior of molecules in different environments.
FAQ Compilation
What factors influence bond angles in molecules?
The primary factor influencing bond angles is the number of electron pairs around the central atom, as described by VSEPR theory.
How can we determine the approximate bond angles in a molecule?
By applying VSEPR theory, we can predict the molecular geometry based on electron pair repulsion, which directly determines the bond angles.
Are there any exceptions to VSEPR theory?
Yes, exceptions arise when steric hindrance or resonance affects the molecular geometry, leading to deviations from the predicted bond angles.